Background
In the era prior to introduction of novel agents, multiple myeloma (MM) harboring t(11;14) was characterized as standard risk. More recently, its unique biology, predictive ability and the prospect of targeted therapeutic agents have renewed interest in t(11;14) MM. Using a large, contemporary real-world database, we investigated the characteristics and outcomes of t(11;14) MM.
Methods
We used the Flatiron Health Electronic Health Record (EHR)-derived de-identified database to source patients (pts) with newly diagnosed MM from 1/2011 to 2/2020 with available Fluorescence in situ Hybridization (FISH) results documented within 90 days of diagnosis. We compared characteristics of t(11;14)+ patients [without additional high-risk FISH abnormalities: del(17p), Ch1 abnormality (Ch1a), t(4;14), t(14;16) or t(14;20)] vs. t(11;14)- patients (without additional high risk FISH) vs. del(17p) (irrespective of other abnormality) vs. Ch1a (Ch1a without additional high-risk FISH) vs. high-risk translocations [t(4;14), t(14;16) or t(14;20) without del(17p)]. We subsequently compared real-world progression-free survival (PFS) and overall survival (OS) across these five subsets. Additionally, we assessed the impact of t(11;14) as additional FISH abnormality in patients with del(17p) and in patients with Ch1a. We used Kaplan Meier methods with log-rank test and Cox proportional hazard regression model for survival analysis with date of diagnosis as the index date for follow-up.
Results
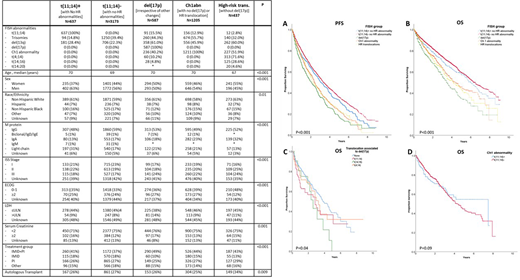
6039 patients in the database met the inclusion criteria. Overall, 83.6% of patients received initial therapy with immunomodulatory agent (IMiD) and/or proteasome inhibitor (PI); of these 40.3% received combination of IMiD and PI. Overall, 27.1% received autologous hematopoietic cell transplantation. Median follow up was 2.1 years (IQR 0.8-4.0). There were 637 pts in t(11;14)+ group, 3173 in t(11;14)- group, 587 in del(17p), 1205 in Ch1a and 437 with high-risk translocations. The t(11;14)+ group had a higher proportion of men, IgM and light-chain isotype, as well as a higher proportion of patients with serum creatinine ³ 2mg/dl (Table). Patients in t(11;14)+ group had worse PFS (mPFS 3.1 vs. 3.3 years, p=0.02) and worse OS (mOS 5.9 vs. 6.5 years , p=0.04) compared to t(11;14)-, but better PFS and OS than the other three high-risk groups (Figure panels A and B). Worse PFS for t(11;14)+ was demonstrable even after adjustment for sex, age, race/ethnicity, immunoglobulin isotype, stage, comorbidities, and treatment received (adjusted HR=0.87, 95% C.I. 0.77-0.98, P=0.027).
We subsequently analyzed the impact of presence of t(11;14) in MM with del(17p) or Ch1a.. The presence of t(11;14) in addition to del(17p) resulted in worse OS compared to del 17p without t(11;14) (mOS 2.8y vs. 3.7y; p=0.04). Indeed, the impact of t(11;14) on del(17p) was comparable to the impact of t(4;14) (Figure, Panel C). There was no difference in survival with concomitant presence of t(11;14) with Ch1a (Figure, Panel D).
Conclusion
MM with t(11;14) has distinct features at presentation and even when treated with modern therapy carries worse prognosis than otherwise standard-risk MM. The concomitant presence of t(11;14) portends a negative prognostic impact to MM with del(17p) but does not appear to impact MM with Ch1a. When present alongside del17p, t(11;14) behaves like a high-risk translocation and identifies a subset of MM in greatest need of newer therapies.
Costa:Amgen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; AbbVie: Consultancy; Celgene: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Genentech: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal